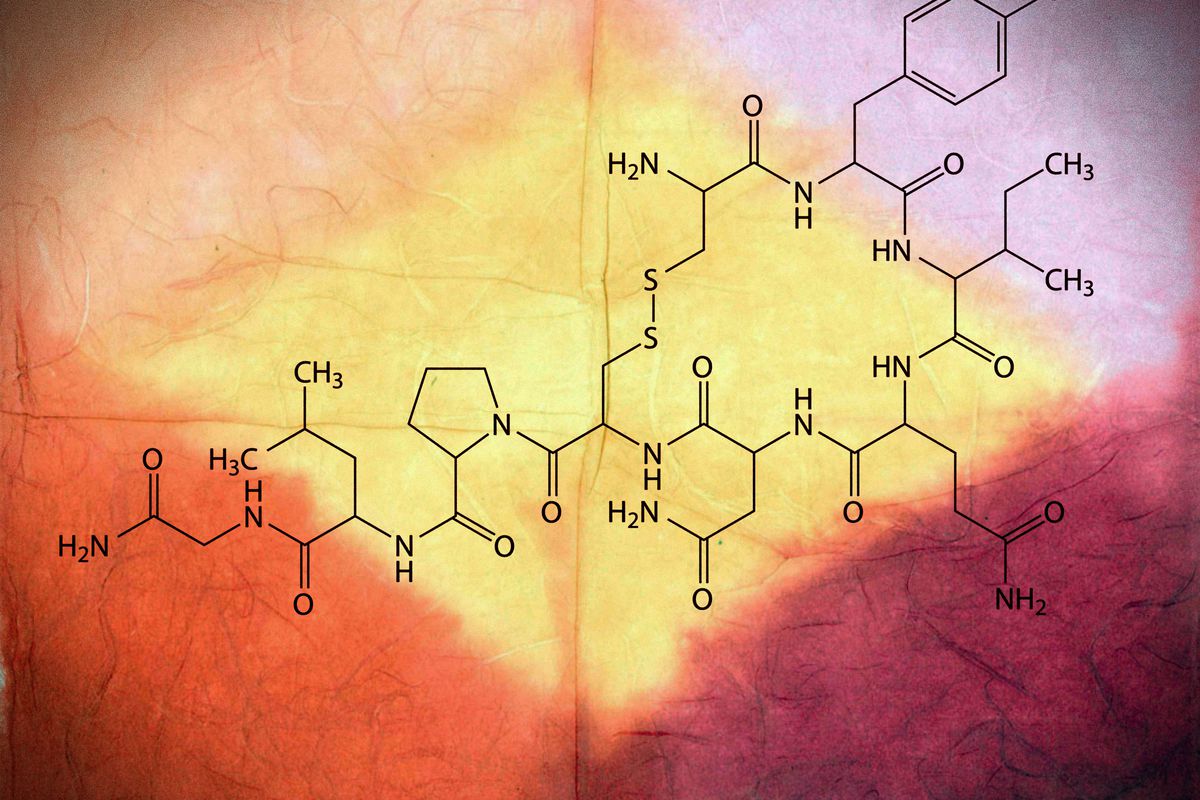
A hormone associated with social bonding led to no beneficiary outcomes for children with autism spectrum disorder, based on experimental tests by a team at Duke University.
First published in the New England Journal of Medicine, the study included almost 300 children diagnosed with autism recruited for a span of six months.
The young participants were administered daily nasal spray doses containing either oxytocin or an inactive ingredient. Although minor improvements in behavior sufficed, none were substantial.
“We conducted a 24-week, placebo-controlled phase 2 trial of intranasal oxytocin therapy in children and adolescents 3 to 17 years of age with autism spectrum disorder,” the authors wrote in their results.
“Of the 355 children and adolescents who underwent screening, 290 were enrolled,” the authors also stated. “A total of 146 participants were assigned to the oxytocin group and 144 to the placebo group; 139 and 138 participants, respectively, completed both the baseline and at least one postbaseline ABC-mSW assessments and were included in the modified intention-to-treat analyses.”
“This is really a major setback. We were really hoping to find a benefit and just couldn’t see it anywhere,” said the study’s lead author in a news release.