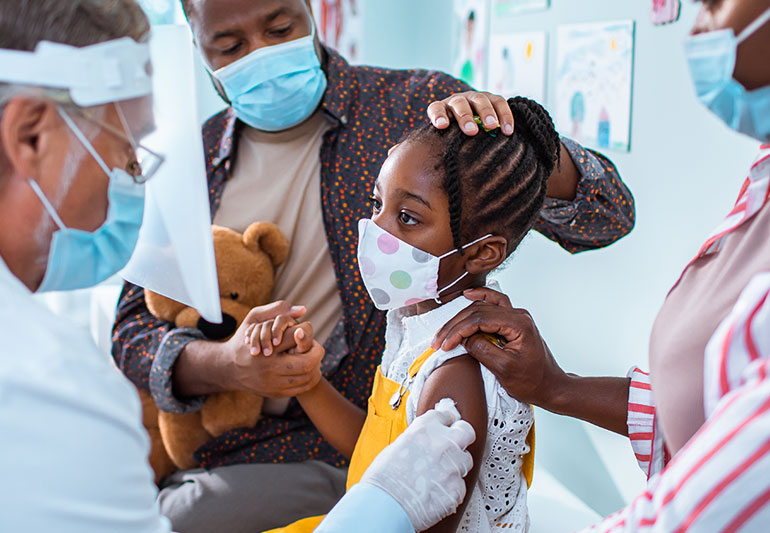
Vaccinations for children aged 5 to 11 have been given the go-ahead by the Food and Drug Administration (FDA), allowing for Pfizer’s vaccines to be distributed and administered.
The new distribution order may place more than 20 million children on the path toward vaccination.
According to a press announcement by the FDA: “Today, the U.S. Food and Drug Administration authorized the emergency use of the Pfizer-BioNTech COVID-19 Vaccine for the prevention of COVID-19 to include children 5 through 11 years of age.”
The news release follows by stating, “The authorization was based on the FDA’s thorough and transparent evaluation of the data that included input from independent advisory committee experts who overwhelmingly voted in favor of making the vaccine available to children in this age group.”
A series of clinical trials have found Pfizer and BioNTech’s COVID-19 vaccine to be around 90 percent effective against the virus among children aged 5 to 11.
Researchers recommend the administration of two separate doses, 3 weeks apart, the FDA report states.